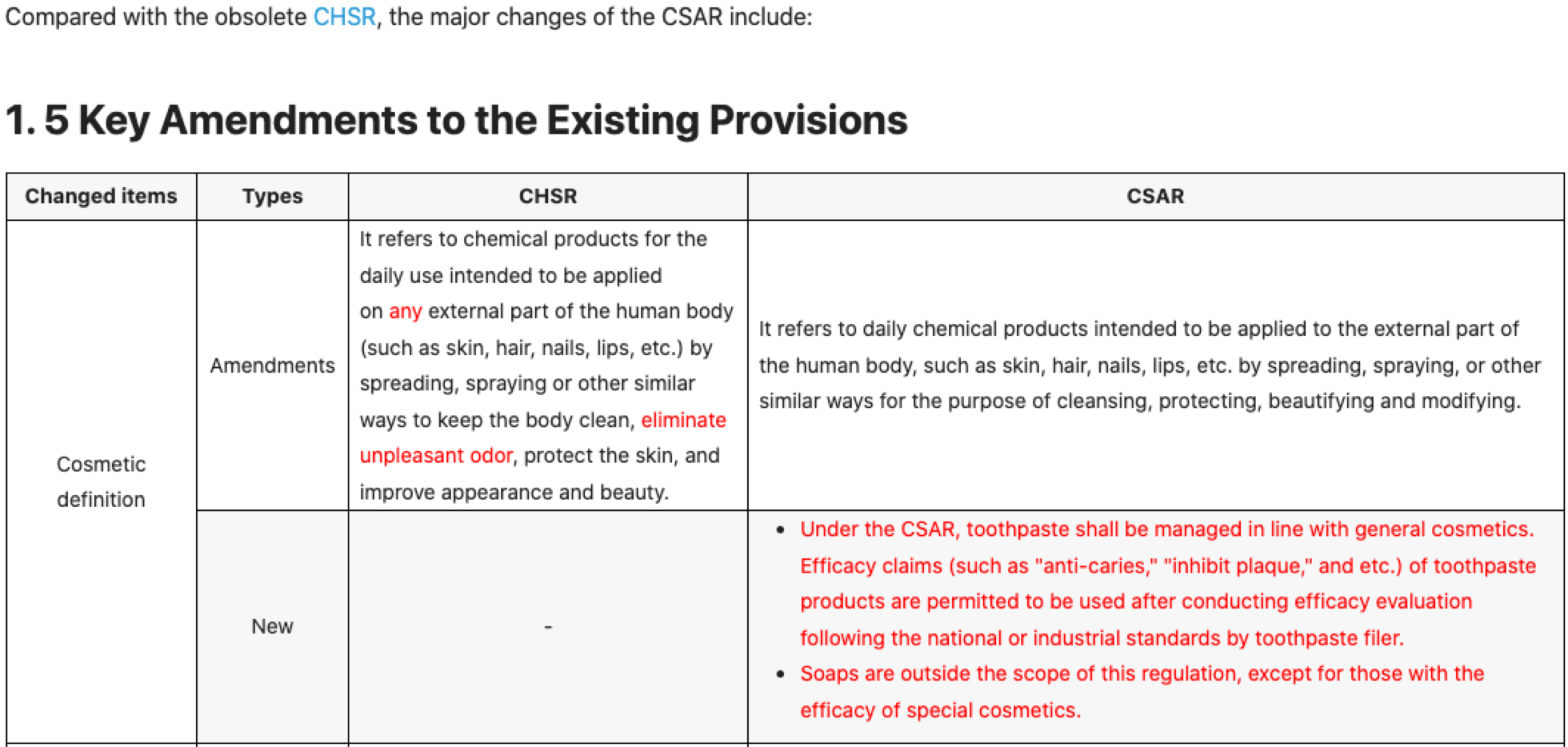
On November 5, 2020, China released the second draft of Guidelines for Cosmetic Efficacy Claim Evaluation (renamed as Standards for Cosmetic Efficacy Claim Evaluation) for public consultation. The Standards clarify the evaluation principles, exemptions, accepted evaluation methods and institutions, obligations of institutions and registrants/notifiers, necessary content in the evaluation report, etc.
It consists of 22 articles and 3 annexes.











 Original regulatory document
Original regulatory document
