On January 10, 2019, National Medical Products Administration released the first collection of answers to the common questions about cosmetics supervision and management. The answers specify that “medicated cosmetics” and “medical skin care products” are banned claims. EGF is also banned from use in cosmetics including any labeling/advertising. Common questions about imported non-special use cosmetics are also answered in the collection.
For Ingredients
1. EGF (Epidermal Growth Factor) is banned in China
Oligopeptide-1 and human oligopeptide-1 (Epidermal Growth Factor, EGF) are not the same substance. Oligopeptide-1 is a synthetic peptide composed of 3 amino acids (glycine, histidine and lysine). Human oligopeptide-1, also known as Epidermal Growth Factor (EGF), is a "53 peptide" consisting of 53 amino acids with a molecular weight of 6,200 Dalton units.
Oligopeptide-1 is included in IECIC 2015, and is generally used as a skin conditioner. Human oligopeptide-1 is not in IECIC 2015 and is generally used in the medical field. The clinical indications are external treatment for burns, wounds and surgical wound healing. EGF can accelerate the growth of transplanted epidermis. Due to the large molecular weight, EGF is more difficult to be absorbed under normal skin barrier conditions. Once the skin barrier is broken, EGF may cause safety problems. So EGF shall not be used as a cosmetic ingredient based on considerations of effectiveness and safety. Any cosmetics using or claiming to use EGF (in Chinese: 人寡肽-1) are illegal products in China.
 (Large)
(Large)
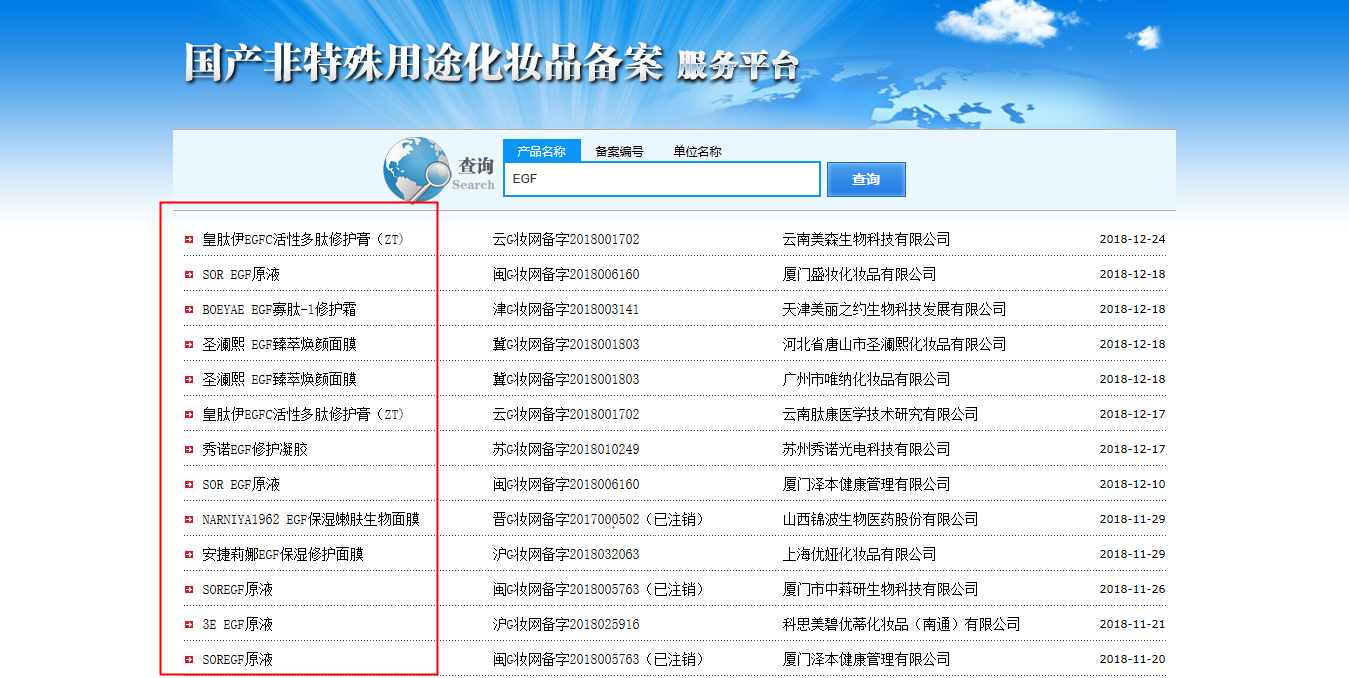
Figure 1: Filed domestic non-special use cosmetics whose names contain "EGF' on NMPA filing system
Among the domestic filed general cosmetics on NMPA filing system, there are still many products’ names contain the word “EGF”. These products’ names are waiting to be modified. Most of the cosmetic products sold in Chinese market claiming EGF (illegal) actually contain oligopeptide-1 (legal).
 (Large)
(Large)
Figure 2: Filing certificate of the product claiming EGF
2. The ingredients such as stabilizers, used to protect other ingredients may not be labeled on the product packaging
A cosmetic ingredient is a component that is purposefully added to the formulation during manufacture process and effective in the final product. In order to ensure the quality of cosmetic ingredients, trace stabilizers, preservatives, antioxidants, etc. do not belong to cosmetic ingredients, may not be marked on the product label but should be reported in the product formulation.
For Cosmetic Claims
Cosmetics are banned from claiming “medicated cosmetics” or “medical skin care products”
Regulations concerning the Hygiene Supervision over Cosmetics 1989 stipulated that cosmetics label, packaging or instructions shall not be marked with indications, shall not claim curative effect, use medical terminology, or advertise medical effects. For products registered or filed as cosmetics, “Medicated cosmetics” (药妆) and “Medical skin care products”(医疗护肤品) are not allowed to be claimed.
Some foreign drugs and quasi-drugs can be used as cosmetics at the same time, but they should be compliant with regulatory requirements for drugs and quasi-drugs in their country of origin, which is much stricter than cosmetics.
For Imported Non-special Use Cosmetics Filing Management
1. There is no validity period for imported non-special use cosmetics, but RP is required to report the production and import of cosmetics, market sales and adverse reactions monitoring condition through online filing system every year.
2. Domestic responsible person intending to import a filed product from more ports outside of designated location is required to supplement relevant information about the new import port and the consignee through online filing system. Import port supplementary does not need review, but if the competent authority finds RP never import products from the new port, the account of RP will be frozen.
3. Oversea cosmetics enterprises may change domestic RP and the scope of the authorized products. If a change is made involving only the scope of authorized products, the RP needs to re-upload the authorization certificate through online filing system. If filed products’ RP changes, the previous and new RP should agree on the liability attribution of the filed product that has been imported and sold. Then the new RP should apply for RP changes through the online filing system and submit the agreement of the previous RP. Changes will be completed after the previous RP confirms the changes through the online filing system.
4. Pursuant to the previous registration requirements, stakeholders with a registration certification can continue to import product within its validity period. But for renewal or change of the registration certification, it is required to apply for filing 5 days before the end of the validity period or before the changed products enter the market. Previous testing report, product safety assessment data for registration can be submitted for filing. If the original documents are submitted for registration, copies with the RP’s official seal are acceptable, accompanied by an explanation.
For more details about cosmetic filing management refer to CL News.





 We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 









