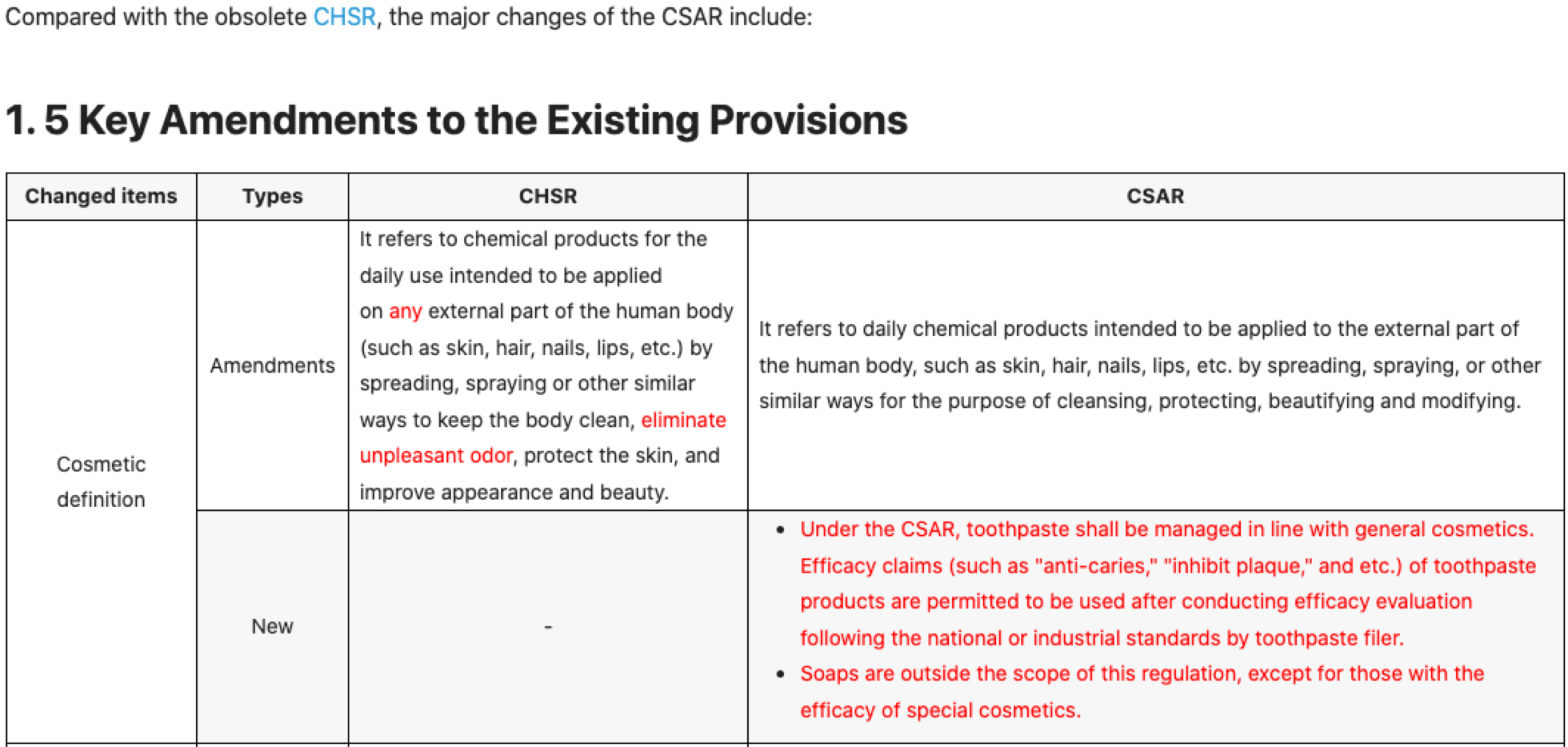
On June 5, 2023, China National Institutes for Food and Drug Control (NIFDC) released nine guidelines pertaining to the registration of Chinese domestic and imported special cosmetics, as well as high-risk new cosmetic ingredients (NCIs). These guidelines provide clear details and time limits for the first registration, registration change, registration renewal and registration cancellation applications, which are highly informative for enterprises seeking guidance.
Guidelines’ Main Content
No. | Guidelines | Main Contents | Attachments |
1 | Guidelines for the Approval of Registration of New Cosmetic Ingredients with High Risks |
| NCI registration and notification information form and its example, basic information of NCI’s R&D report and its example, NCI’s usage information and its example, technical requirements for NCI and its example, flowchart for NCI registration, NCI’s registration certificate, the template of decision letter on not approving administrative licensing for NCI |
2 | Guidelines for the Approval of Registration Change of Chinese Domestic Special Cosmetics | Application form for registration change of special cosmetics and its example, handling process, example of the registration certificate of Chinese domestic special cosmetics, the template of decision letter on not approving registration change | |
3 | Guidelines for the Approval of Initial Registration of Chinese Domestic Special Cosmetics | Cosmetics registration and notification information form and its example (applicable for Chinese domestic cosmetics and Chinese domestic registrant/notifier), cosmetic product naming basis and its example, formula table and its example, cosmetic executive standard and its example, cosmetic labeling manuscript and its example, cosmetic product’s safety assessment report and its examples (full version and simplified version), handling process, example of Chinese domestic special cosmetics’ registration certificate, the template of decision letter on not approving administrative licensing for special cosmetics | |
4 | Guidelines for the Approval of Registration Renewal of Chinese Domestic Special Cosmetics | Cosmetic product registration renewal application form and its example, the self-inspection report on cosmetic registration renewal and its example, handling process, example of Chinese domestic special cosmetics registration certificate, the template of decision letter on revoking cosmetic administrative license | |
5 | Guidelines for the Approval of Registration Cancellation of Chinese Domestic Special Cosmetics (Cancellation upon Application) | Cosmetic registration cancellation application form and its example, handling process, the template of decision letter on cancellation approval | |
6 | Guidelines for the Approval of Registration Change of Imported Special Cosmetics | Application form for registration change of special cosmetics and its example, handling process, example of registration certificate of imported special cosmetics, the template of decision letter on not approving registration change | |
7 | Guidelines for the Approval of Initial Registration of Imported Special Cosmetics | Cosmetics registration and notification information form and its example (applicable for imported cosmetics and Chinese domestic registrant/notifier), cosmetic product naming basis and its example, formula table and its example, cosmetic executive standard and its example, cosmetic labeling manuscript and its example, cosmetic product’s safety assessment report and its examples (full version and simplified version), handling process, example of imported special cosmetics registration certificate, the template of decision letter on not approving administrative licensing for special cosmetics | |
8 | Guidelines for the Approval of Registration Renewal of Imported Special Cosmetics | Cosmetic product registration renewal application form and its example, the self-inspection report on cosmetic registration renewal and its example, handling process, example of imported special cosmetics registration certificate, the template of decision letter on revoking cosmetic administrative license | |
9 | Guidelines for the Approval of Registration Cancellation of Imported Special Cosmetics (Cancellation upon Application) | Cosmetic registration cancellation application form and its example, handling process, the template of decision letter on cancellation approval |








 Original regulatory document
Original regulatory document
