Editor's Notes: This article was originally published on June 15, 2023, and was updated on November 7, 2023, as per the latest developments. The updated contents are marked in red below.
On June 13, 2023, EU Scientific Committee on Consumer Safety (SCCS) initiated a ten-week public consultation on the preliminary opinion concerning Benzyl Salicylate (CAS No. 118-58-1). Following the consultation period, SCCS published the final opinion on November 6, 2023, which reaffirms the same conclusion.1
Benzyl Salicylate commonly serves a perfuming function in cosmetics. As an established contact allergen in humans, it is a restricted ingredient currently regulated under Regulation (EC) No 1223/2009 (Cosmetics Regulation), and subject to individual labelling requirement. To comply with this requirement, its presence shall be indicated in the ingredients list on product packaging when its concentration exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
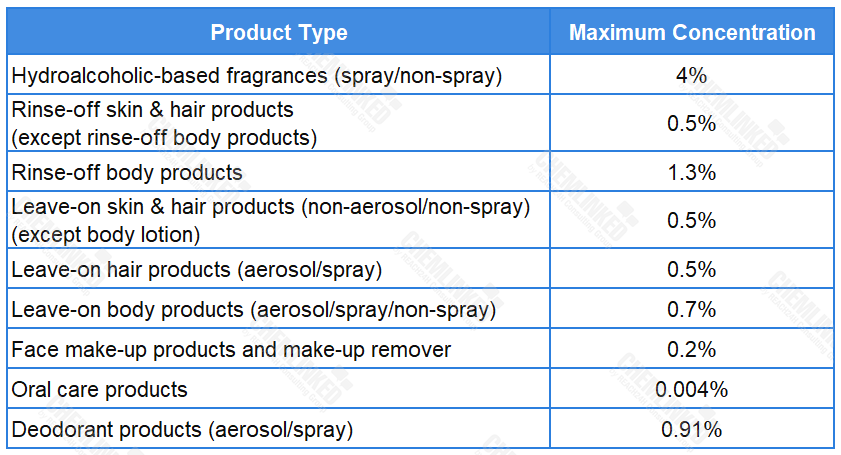
In addition, Benzyl Salicylate was included in the European Commission’s priority list of potential endocrine disruptors (EDs) due to concerns about its adverse effects. Following a public call for data and information, SCCS conducted a safety assessment on it, and concluded that the use of Benzyl Salicylate is safe in cosmetics when being used up to the maximum concentrations specified as follows:



 We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 










