On Nov 6, 2012, SFDA published a notice to add a new conclusion of technical review on special use cosmetics, which will enter into force as of Jan 1 2013 (Chemlinked August 22).
According to the article 17,18,19 in Key Points for Technical Review of Cosmetics, three possible conclusions will be made:
1) Suggest to be approved:
Generally, when the product is reviewed and assessed as in accordance with the requirements of regulations and technical standards and no problems are identified.
2) Suggest for further review after documents supplementation:
- Content of dossier need to be revised or supplementary data is needed
- A verification test is needed
- An explanation or supporting documents is needed from a third party
3) Suggest to be disapproved:
- The dossier contents or product samples are false
- The formula ingredient doesn’t meet requirements
- The test result doesn’t meet safety requirements
- The supplementary data is not consistent with previously submitted technical information.
This newly-added conclusion is “Suggest to be approved after documents improvement”, if the product Chinese name or Pinyin needs to be modified; description, expression, format and writing of submitted documents shall be further improved, which are all minor discrepancies. As long as they are corrected and in compliance with relevant requirements, they can be approved. Therefore, the conclusion can be categorized between the conclusion 1) and 3).
The addition of the new technical review conclusion contributes to enhancing the licensing efficiency and speeding up the approval of some cosmetics.
The technical review is just one step of the administrative licensing. There are three departments under SFDA working on the administrative licensing of cosmetics, as shown in Figure 1.
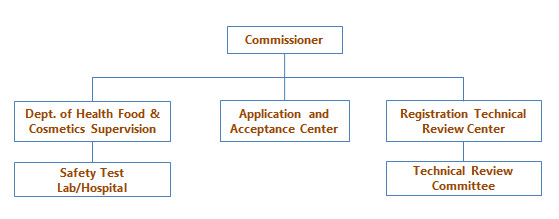
Figure 1: Departments in charge of administrative licensing of cosmetics
After applicants submit all registration documents and required samples, it takes 5 days for Application and Acceptance Center to perform a format check on all submitted documents and issue an acceptance notice, 90 days for Registration Technical Review Center to conduct the technical review of the product and 20 days for the Department of Health food & Cosmetic Supervision to issue an approval notice.
Among the previous three steps, the technical review is of critical importance in determining whether a product can be approved.
The Cosmetic Registration Review Center will organize technical review meetings in the late of every month, usually lasting one week; provide technical consultation to cosmetic producers as well as offer review opinion and conclusion for SFDA’s final approval. Meanwhile, it plays a very important role in formulating and drafting new regulations. For example, at the request from SFDA, it involved in the amendment of the cosmetic overriding “Regulations concerning the Hygiene Supervision over Cosmetics” (Chemlinked Sept 26).
Specifically, the Technical Review Committee consists of one chairman, two vice chairmen, two secretaries and four expert groups to review product name/label, product formula, micro and chemical data along with toxicology data respectively. Those experts are selected from institutes of drug control, centers for disease control and prevention, hospitals and colleges, who have rich experience in cosmetic testing and inspection.
The procedure of technical reciew is shown as below.
During the process of review, Mr Liu Baojun, the Director of Cosmetic Review Division, reminds us the following aspects:
- Product label and name: the applicant shall concentrate on mandatory labeling items, warning statement, authentic Chinese translation of imported product label, appropriate efficacy claims and the correct SPF identification.
- Product formula: no prohibited ingredients list in Hygienic Standard for Cosmetic (2007) shall be used; cosmetics for hair growing, sliming and beautifying breast need the description of efficacy ingredients and their use references.
- Hygienic chemical and microbiological test: all testing results of heavy metals, microorganism, restricted and efficacy ingredients must meet relevant up-limits requirements and are in line with formula statement.
- Toxicological safety data review: the product should not indicate obvious skin and eye irritation or erosion and present no obvious skin allergic reaction and /or photo toxicity; Infant or children products will be subjected to higher safety standard.
- Human safety evaluation: the animal test must be conducted prior to the cosmetic human test; there shall be no obvious adverse reaction to human skin after human using test or human patch test.



 We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 







