Launched in 2005, the EU Rapid Exchange of Information System (RAPEX) is a Europe-wide rapid alert system that enables Member States to share measures taken against dangerous non-food products. Whenever a product found to pose serious risks to consumer's health and safety, the national authorities of the Member States will send alerts through the system, then a dedicated team in the European Commission will circulate them through the online portal on a daily basis.
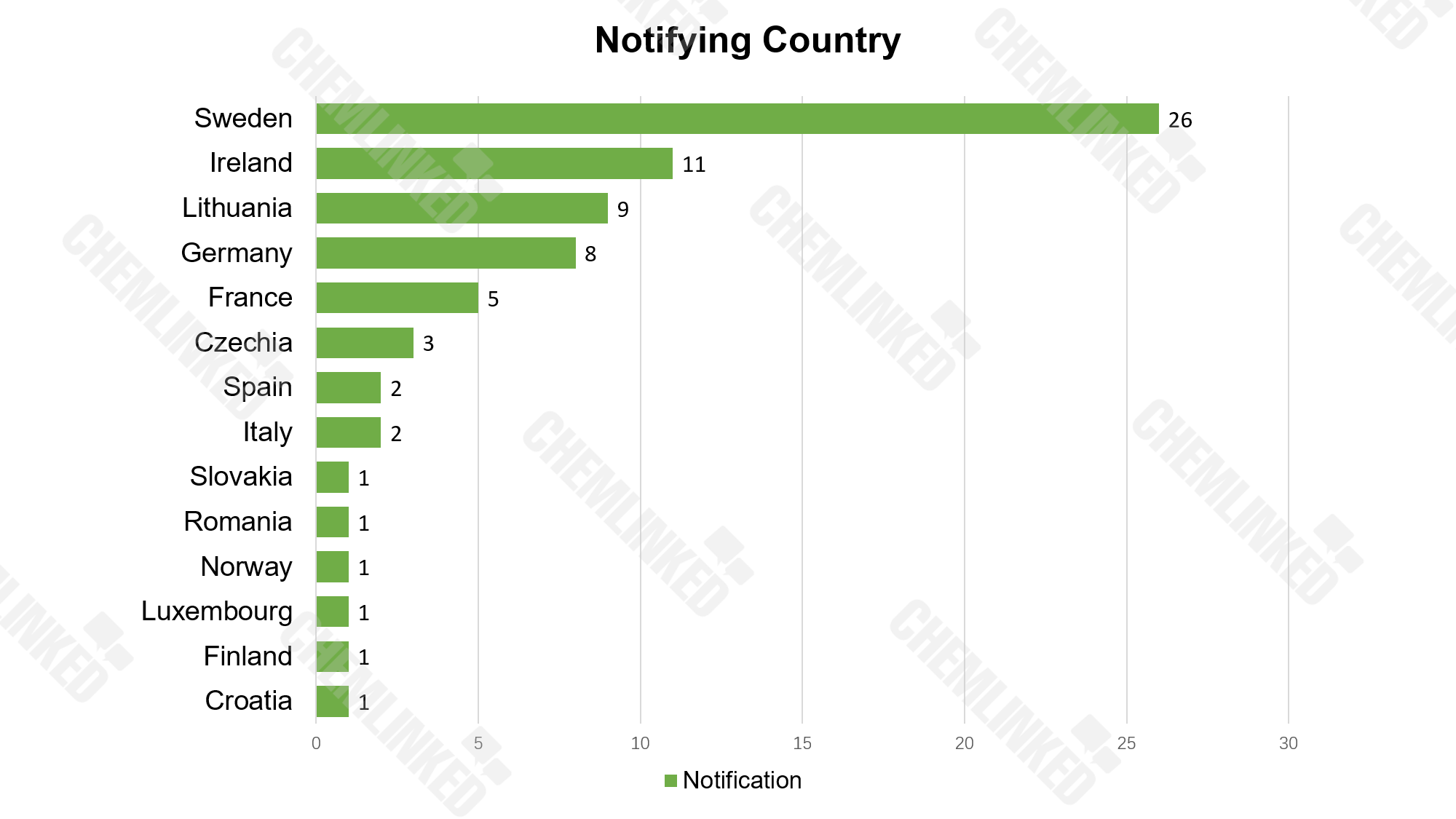
From January to June 2022, RAPEX received 72 alerts on measures taken against non-compliant cosmetics. These cosmetics were notified by 14 countries, among which Sweden, Ireland, Lithuania, Germany and France ranked the Top 5.
For the notified cosmetics, the detection of prohibited ingredient (45%) is the primary non-compliance reason, followed by other reasons such as failing to meet the use condition (26%) and risk of being contaminated (20%).

Noteworthy Compliance Requirements for the Above-mentioned Notified Cosmetics
1. Ingredient
Regulation (EC) No 1223/2009 (Cosmetics Regulation) lays down rules regarding the use of ingredients in cosmetics. In its Annex II, it compiles ingredients prohibited for use in cosmetics. Cosmetic products available on the EU market shall not contain any ingredient listed in this Annex.
In 2022 H1, there are 33 alerts for cosmetics containing prohibited ingredients. The detected ingredients in these alerts include:
| No. | Detected Prohibited Ingredient | Risk |
| 1 | Barium Peroxide | Barium Peroxide releases Barium, which can be easily absorbed and has toxic effects on the body impairing muscle, heart and kidney activity, leading to arrythmia, paralysis or gastrointestinal alterations. |
| 2 | Clobetasol Propionate | Clobetasol is a corticosteroid that should be applied only if medically prescribed. Exposure to it could cause skin irritation and may lead to endocrine problems. |
| 3 | Mometasone | Mometasone is a corticosteroid that should be made available only under medical prescription: exposure to it could cause skin irritation and may lead to endocrine problems. |
| 4 | Hydroquinone | Hydroquinone can cause skin irritation and dermatitis. Note: Hydroquinone is prohibited in cosmetics with the exception of its professional use in artificial nail systems. |
| 5 | Butylphenyl Methylpropional (BMHCA) | BMHCA may harm the reproductive system, may harm the health of the unborn child and may cause skin sensitisation. |
| 6 | Formaldehyde | Formaldehyde is a skin sensitiser and can trigger allergic reactions and may cause cancer. |
| 7 | Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde | Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde is a skin sensitiser that triggers allergic reactions or contact dermatitis. |
| 8 | Betamethasone | Exposure to Betamethasone could cause skin irritation and may lead to endocrine problems. It should be made available only under medical prescription. |
| 9 | Isobutylparaben | Isobutylparaben might be toxic for reproduction and development, and might specifically be harmful to the male reproductive system. |
| 10 | Cloprostenol Isopropyl Ester | Cloprostenol Isopropyl Ester can cause eye and ophthalmic defects and should not be used by pregnant and breastfeeding women, as it may affect the child. It should be used under supervision of an ophthalmologist. |
| 11 | 2-Chloroacetamide | Dermal contact with 2-Chloroacetamide containing products is suspected of damaging fertility and may cause an allergic skin reaction. |
2. Use Condition
In addition to the prohibited ingredients, Cosmetics Regulation also compiles ingredients that can be used if specific use conditions are met. Prior to placing a cosmetic product on the market, the responsible person shall ensure the product comply with the specified use conditions of its ingredients.
Among the cosmetics notified in 2022 H1, 26% of them failed to meet the use conditions stipulated in Cosmetics Regulation. ChemLinked has sorted out the ingredients involved in these non-compliance cases, and detailed their corresponding use conditions in the table below:
| No. | Ingredients | Use Conditions |
| 1 | Methylchloroisothiazolinone and Methylisothiazolinone (MCI and MI) | MCI and MI can be used only in rinse-off products. |
| 2 | Thioglycolic Acid | The maximum concentration of Thioglycolic Acid in cosmetics is:
|
| 3 | Propylparaben | Propylparaben is forbidden in leave-on products designed for application on the nappy area of children under three years of age. |
| 4 | Limonene, Linalool, Benzyl Alcohol, Citronellol, Eugenol, Citral, Cinnamal, Benzyl Benzoate and Geraniol | The presence of these fragrances must be indicated in the list of ingredients when their respective concentration exceeds:
|
| 5 | Hydrogen Peroxide | The maximum concentration of Hydrogen Peroxide in cosmetics is:
|
| 6 | p-Phenylenediamine | When p-Phenylenediamine is used as a hair dye substance in oxidative hair dye products, after mixing under oxidative conditions, its maximum concentration applied to hair must not exceed 2% calculated as free base. |
| 7 | Salicylic Acid | The maximum concentration of Salicylic Acid in cosmetics is:
|
On the EU Market, there are also cosmetics containing ingredients that are not taken up in the Annexes to Cosmetics Regulation. To protect consumers from allergy or skin sensitisation, prior to placing their cosmetics on the market, the responsible persons shall ensure that the products have undergone safety assessments for the use of any ingredient contained.
3. Microbiological Specification
Microbiological contamination refers to the unwanted presence of microorganisms in products. Exposure to the microbiologically contaminated products may pose a risk of skin infection, in particularly to sensitive consumers. According to The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, the microbiological quality of the finished cosmetic product needs to meet the specifications below:
| Types of microorganism | Products specifically intended for children under three years of age, the eye area or the mucous membranes | Other products |
| Total Aerobic Mesophilic Microorganisms (Bacteria plus yeast and mould) | ≤ 1 x 102 CFU per g or mla | ≤ 1 x 103 CFU per g or mlb |
| Escherichia coli | Absence in 1g or 1ml | Absence in 1g or 1ml |
| Pseudomonas aeruginosa | Absence in 1g or 1ml | Absence in 1g or 1ml |
| Staphyloccocus aureus | Absence in 1g or 1ml | Absence in 1g or 1ml |
| Candida albicans | Absence in 1g or 1ml | Absence in 1g or 1ml |
| Notes: 1) Due to inherent variability of the plate count method, according to US Pharmacopoeia Chapter 61 or European Pharmacopoeia Chapter 2.6.12, Interpretation of results, results considered out of limit if: a > 200 CFU/g or ml, b > 2,000 CFU/g or ml. 2) When colonies of bacteria are detected on Sabouraud Dextrose agar, Sabouraud Dextrose agar containing antibiotics may be used. | ||
4. Heavy Metal Specification
Heavy metals are banned in cosmetics under Cosmetics Regulation, while traces are allowed if the concentration level will not pose a risk to human health. However, there are no specific limits regarding the trace concentration established in Cosmetics Regulation. As a source of reference, the Member State Germany sets the limits (mg/kg) of unavoidable metal content in cosmetics, respectively:
cadmium: 0.1;
mercury: 0.1;
antimony: 0.5;
arsenic: 0.5 (or 2.5 for theatre make-up); and
lead: 2.0 (or 5.0 for certain make-up products) and 0.5 (toothpaste).



 We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 









