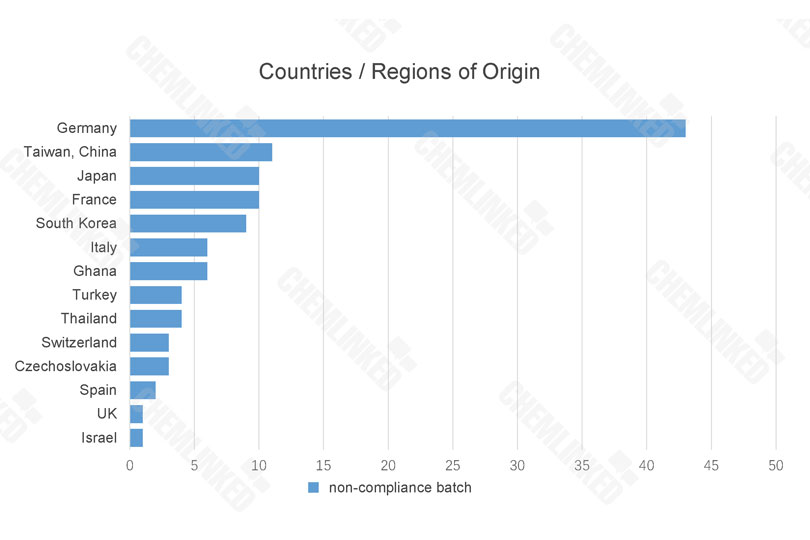
From January to December 2021, China General Administration of Customs P.R.C. (GACC) revealed a total of 113 batches of rejected cosmetics. 1 These rejected cosmetics were imported from 14 countries and regions, among which Germany, Taiwan of China, France, Japan and South Korea ranking the Top 5.
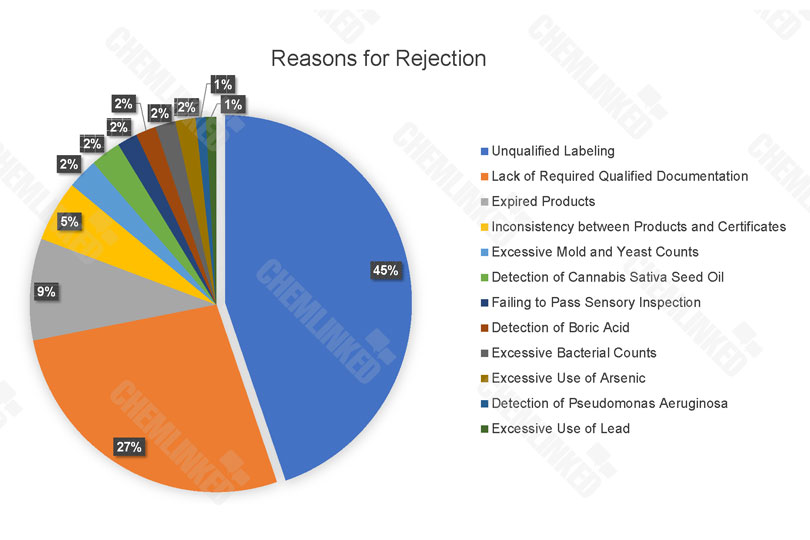
For the rejected cosmetics, unqualified labeling (45%) is the primary reason for rejection, followed by other reasons such as lack of required qualified documentation (27%), expired products (9%) and inconsistency between products and certificates (5%).
Corresponding Compliance Requirements for the Above-mentioned Rejected Cosmetics
1. Labeling
The imported cosmetics can be either packed in the original package with a compliant Chinese sticker affixed to its original label, or in a package specifically designed for the Chinese market. The label on the package shall conform to relevant laws, administrative regulations, and the mandatory requirements in China's national technical standards.
It is worth noting that, Administrative Measures on Cosmetics Labeling, the new regulation specifying detailed requirements for cosmetic labeling and claims, will be effective on May 1, 2022. Cosmetic manufacturers shall meet the requirements stipulated in this regulation after the effective date as well.
2. Documentation
For the on-site inspection on imported cosmetics, GACC shall check the conformity of products and corresponding certificates, product sensory properties and sanitary conditions of transportation, etc. Cosmetics importers are required to prepare the documentation in accordance with Administrative Measures on Inspection and Quarantine of Import and Export Cosmetics (2018).
Article 39 of Cosmetic Supervision and Administration Regulation stipulates that, cosmetics producers and operators shall store and transport cosmetics in accordance with the provisions of relevant laws and regulations, and regularly inspect and promptly dispose of deteriorated and expired cosmetics.
4. Microbiological and Metal Specification
According to the Safety and Technical Standards for Cosmetics 2015 (STSC 2015), the bacterial counts shall be:
no more than 500 CFU/g or 500 CFU/ml for eyes make-up, lips make-up and children cosmetics;
no more than 1,000 CFU/g or 1,000 CFU/ml in other cosmetics.
As stipulated in STSC 2015 as well, the mold and yeast counts shall be no more than 100 CFU/g or 100 CFU/ml in cosmetics, and pseudomonas aeruginosa shall not be detected.
For metal contents, the limitation for arsenic and lead in cosmetics is 2 mg/kg and 10 mg/kg respectively.
5. Ingredient
On May 28, 2021, National Medical Products Administration (NMPA) implemented the finalized Inventory of Prohibited Ingredients for Cosmetics and Inventory of Prohibited Plant (Animal) Ingredients for Cosmetics. In these two inventories, 24 prohibited cosmetic ingredients were newly added, including boric acid and cannabis sativa seed oil. According to NMPA, from May 28, 2021, it is not allowed to produce or import products containing the prohibited ingredients stipulated in the new inventories. 2
Notes: all non-compliant cosmetics have been either returned or destroyed at the port.






 We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 







