Top 5 Crucial Cosmetic Regulatory Updates
1: Indonesia Implements Criteria and Procedure for Submission of Cosmetics Notification
In Indonesia, both domestically manufactured and imported cosmetics should go and notify the Agency for Drug and Food Control (BPOM) before being circulated on the market. On Jun. 24, 2020, Indonesia implemented the amended Criteria and Procedure for Submission of Cosmetics Notification. The new regulation clarifies terms, the scope of notification applicants, dossiers necessary, notification procedures, notification templates, cosmetics categories, etc.
Only eligible applicants can apply for a notification.
Cosmetics industry in Indonesia;
An individual business/business entity in the cosmetics field that makes a production contract with the cosmetics industry in Indonesia;
Cosmetics importers (including an individual business/business entity that makes a production contract with the cosmetic industry outside Indonesia). [Read More]
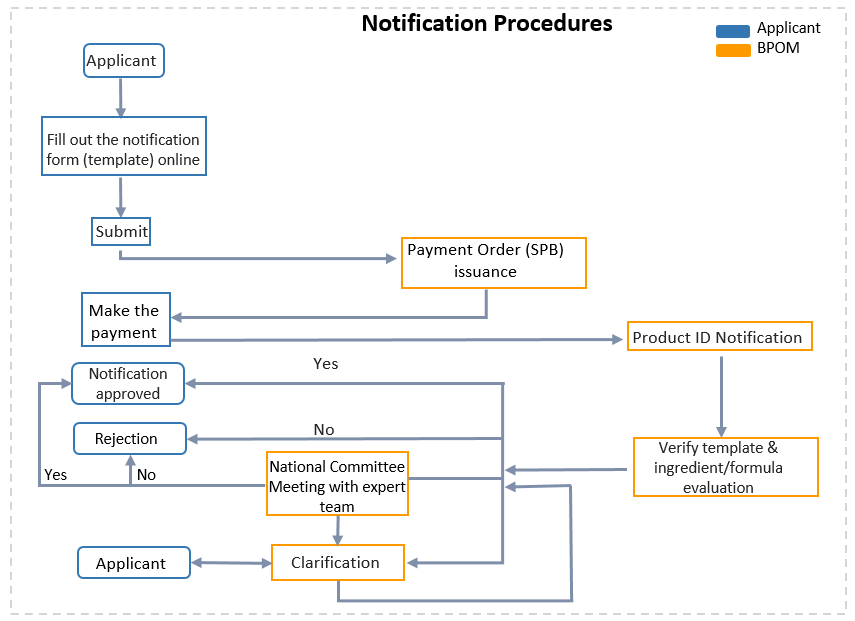 Indonesia Cosmetic Notification Procedures, Designed by ChemLinked
Indonesia Cosmetic Notification Procedures, Designed by ChemLinked
2: Indonesia Clarifies Standards for Permitted and Prohibited Cosmetic Claims
Before conducting cosmetic notifications in Indonesia, business operators must ensure the objectivity and authenticity of product claims. After obtaining a notification certificate, the notification certificate holders should be responsible for claims compliance.
On Dec. 11, 2020, Indonesia released the draft Technical Requirements for Cosmetics Claims. The draft regulation clarifies requirements for cosmetic claims on labeling and advertising and attaches two lists of prohibited and permitted claim examples.
Cosmetic claims must meet the following criteria:
Legal compliance;
Truth;
Honesty;
Justice;
Verifiable;
Clear and easy to understand;
Cannot claim to be medicine. [Read More]
3: Indonesian Halal Certification Process Changes Following the Pass of the Omnibus Law
Indonesian Omnibus Law was signed by President Jokowi on Nov. 2, 2020. The amendments of 76 existing laws (including Halal Product Assurance Law) aim to reduce bureaucracy and boost investment in Indonesia.
The main amendments include:
1) The time required from application to halal certificate issuance has been shortened from more than three months to around 21 working days.
 Indonesia Cosmetic Halal Certification Procedures, Designed by ChemLinked
Indonesia Cosmetic Halal Certification Procedures, Designed by ChemLinked
2) Exempts the halal certification fees for micro and small businesses. And the BPJPH can renew the halal certificate directly if the business operator states that it fulfills the halal production process requirements and does not change the ingredients.
3) The Omnibus Law partially resolves the ambiguity on the responsibility division between BPJPH and MUI. MUI is authorized for the product halal status determination through a halal fatwa. BPJPH will coordinate with MUI about the product halal status determination. [Read More]
4: Indonesia Implements Regulation for Supervision on Manufacture and Circulation of Cosmetics 2020
On Feb. 5, 2020, Indonesia implemented amended Regulation for Supervision on Manufacture and Circulation of Cosmetics. The regulation revises the definitions of Manufacture and Circulation, clarifies the responsibilities of notification number owners and distribution business operators, supervision targets, items, and methods. Supervision of cosmetics manufacture and distribution is carried out through inspection of facilities and cosmetic products. [Read More]
Targets | Coverage | Items | |
Facilities | Facilities of notification number owner | Cosmetics industry |
|
Importers engaged in the field of cosmetics |
| ||
Individual business or business entity in the field of cosmetics who entered into a production contract | / | ||
Distribution facilities |
| / | |
Cosmetic products | / |
| |
5: Indonesia Clarifies GMP Certification Procedures
Cosmetic enterprises in Indonesia in conducting production activities are obliged to comply with GMP Guideline. On Dec. 1, 2020, Indonesia BPOM released a new draft regulation, Certification Procedures of Cosmetic Good Manufacturing Practices, clarifying the types of GMP certification documents, the required documents for GMP certification, the certification process, certification renewal, and modification.
Enterprises contracted to produce cosmetics need to prove their GMP implementation status with a GMP Certificate. Enterprises that have not yet been contracted to produce cosmetics need to prove their GMP compliance status with a GMP Certificate or a Fulfillment Certificate for GMP Aspects. [Read More]
Other Notable Regulations/Drafts Issued in 2020
Regulation | Effective/Release Date | Main Contents | Further Reading |
Effective: Jun. 29, 2020 | According to the Regulation for Import of Complementary Goods, Goods for the Need of Market Test, and After Sales Service 2015, business operators with API-P can import finished cosmetics when they serve as complementary goods and goods of market test and/or after-sales service. However, under this amendment, finished cosmetics are allowed to be imported as complementary goods, but not allowed to be imported as goods for market testing or after-sales service. | ||
Amendments to Regulation for Supervision on the Entry of Drug and Food into the Territory of Indonesia and Regulation for Supervision on the Entry of Drug and Food Ingredients into the Territory of Indonesia | Effective: Jul. 13, 2020 | The regulations stipulate that imported cosmetic products and ingredients must obtain a SKI Post Border before entering the Indonesian territory. The amendments clarify the obligations of SKI Post Border applicants and adjust the document requirements. | |
Release: Aug. 7, 2020 | The amendments added several types of cosmetics prohibited from being manufactured as well as permitted to be manufactured by manufacturers with class B production licenses. | ||
Release: Aug. 4, 2020 | This regulation serves as a guide for business actors obtaining licensing (including notification certificate and GMP certificate) in the cosmetic sector. It clarifies the certificate types, requirements, procedures, validity period, etc. | ||
Regulation for the Determination of Forms of Cosmetic Preparations | Effective: Sep. 28, 2020 | This regulation is formulated to facilitate the determination of cosmetic types in public services, especially GMP certification. Types of cosmetics intended to be produced must be stated on the GMP Certificate or Fulfillment Certificate for GMP Aspects. Cosmetics enterprises are prohibited from producing cosmetics outside the types stated in the GMP Certificate or Fulfillment Certificate for GMP Aspects. | |
Amendments to Guideline for Cosmetic Good Manufacturing Practice (Draft) | Release: Oct. 2020 | The draft amendments include the introduction of Gradual Fulfillment Certificate for GMP Aspects and the inclusion of GMP Guideline operational instructions. |



 We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global cosmetic market entry services (including cosmetic registering & filing, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 









