On 1st July 2019, the overarching cosmetic regulation “Cosmetic Hygiene and Safety Act” in Taiwan entered into force, replacing the former one “Statue for Control of Cosmetic Hygiene”. The categories of cosmetics changes from general cosmetics and medicated cosmetics in the old regulation to general cosmetics and specific purpose cosmetics in the regulation.
The newly enforced cosmetic hygiene and safety act establishes a completely new administrative system including a new notification system and requires the establishment of a PIF (product information files). Starting from the effective date (1st July, 2019), all general cosmetics can start their notification procedure through an online platform.
The actual implementation date for general cosmetic notification is 1st July, 2021. After that, cosmetic products without notification will be regarded as illegal. The notification functions as a record to help TFDA to assign liability in case of any safety problems. Specific purpose cosmetics are still subject to registration, but 5 years later (1st July, 2024), they will be subject to a notification and PIF system as well. TFDA has also strengthened provisions related to post-market surveillance. In addition, the registration of colorants has been abolished.
Part 1 Regulatory Framework and Competent Authority
1 Existing Key Cosmetic Regulations in China's Taiwan
Type | Regulation | Release Date | Implementation Date |
Overarching | 2018-05-02 | 2019-07-01 | |
2019-06-27 | 2019-07-01 | ||
Manufacture | 2019-08-13 | 2019-07-01 | |
2019-08-29 | 2019-07-01 | ||
| Product Approval | 2019-05-30 | 2019-07-01 | |
Regulations for Cosmetic Product Information File Management | 2019-05-30 | 2019-07-01 | |
Regulations for Issuance of License of Specific Purpose Cosmetics | 2019-05-28 | 2019-07-01 | |
Labeling/claim | Labeling Requirements for Cosmetic Packaging, Containers, Labels or Directions | 2019-05-30 | 2021-07-01 |
2019-06-04 | 2019-07-01 | ||
Post-market surveillance | Regulations Governing the Source and the Flow Data of Cosmetic Products | 2019-05-22 | 2019-07-01 |
Regulations for Reporting Cosmetics Serious Adverse Effects and Hazards to Hygiene and Safety | 2019-05-22 | 2019-07-01 | |
2019-05-22 | 2019-07-01 | ||
Regulations for the Inspection and Examination of Imported Cosmetics | 2019-06-27 | 2019-07-01 | |
Regulations on Cosmetic Hygiene and Safety Violation Report and Reward | 2019-06-27 | 2019-07-01 | |
Testing | 2019-06-28 | 2019-11-09 |
2 Cosmetic Competent Authorities
"Taiwan Food and Drug Administration", a sub department of the Ministry of Health and Welfare oversees comprehensive supervision on cosmetics, including formulating and releasing cosmetic regulations, governing the notification of cosmetic products, issuing specific purpose cosmetic licenses and etc.
Part 2 Cosmetic Products
1 Definition
The "Cosmetic Hygiene and Safety Act" stipulates that "Cosmetics" means the substance(s) for external use on the human body to freshen the hair or skin, to stimulate the sense of smell, to cover body odor, or to improve facial appearance. Taiwan released a List of Scope and Categories of Cosmetics to define the categories of cosmetics.
2 Classification
Taiwan regulates cosmetics based on functions. Cosmetic products are categorized into general cosmetics and specific purpose cosmetics. The old name "medicated cosmetics" has been abolished. Specific purpose cosmetics include:
sunscreens
hair dyes
hair perms
deodorants
teeth whitening products
cosmetics with other purposes
The remaining products in the Scope and Categories of Cosmetics refer to general cosmetics.
3 Obligations of Manufacturers and Importers
Type | Obligation |
General Cosmetics | 1) Notification l July 1, 2019-July 1, 2021 (grace period): same as before, no need to notify cosmetics l From July 1, 2021: notify cosmetics 2) Establish PIF l July 1, 2019-July 1, 2025 (grace period): no need to establish PIF l From July 1, 2025: establish PIF for baby, lip and eye products and unmedical toothpaste and mouthwash l From July 1, 2026: establish PIF for other general cosmetics (solid handmade soap excluded) |
Specific purpose cosmetics | 1) Registration l July 1, 2019-July 1, 2024: same as before, register cosmetics 2) Notification & Establish PIF l From July 1, 2024: change registration to notification and establishment of PIF |
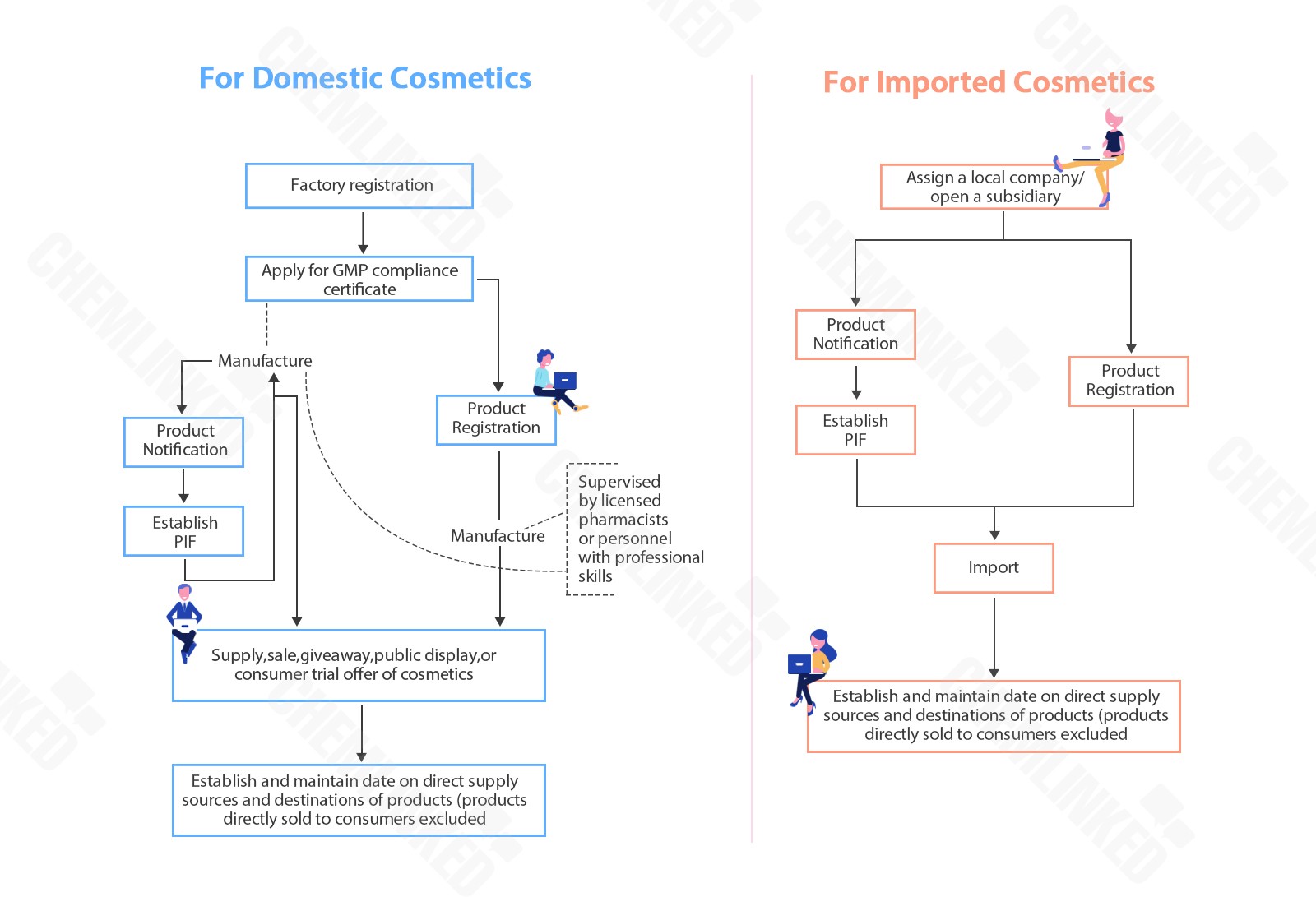
Note: Registration of specific purpose cosmetics will be changed to notification and establishment of PIF from July 1, 2024.
The regulatory trend in Taiwan is to unify the management of general cosmetics and specific purpose cosmetics, adopting an administrative system of notification and establishment of a PIF (product information files) similar to EU and ASEAN. To help the cosmetic industry better prepare for the system, the TFDA granted a grace period for both general cosmetics and specific purpose cosmetics, shown in the table above.
Notification
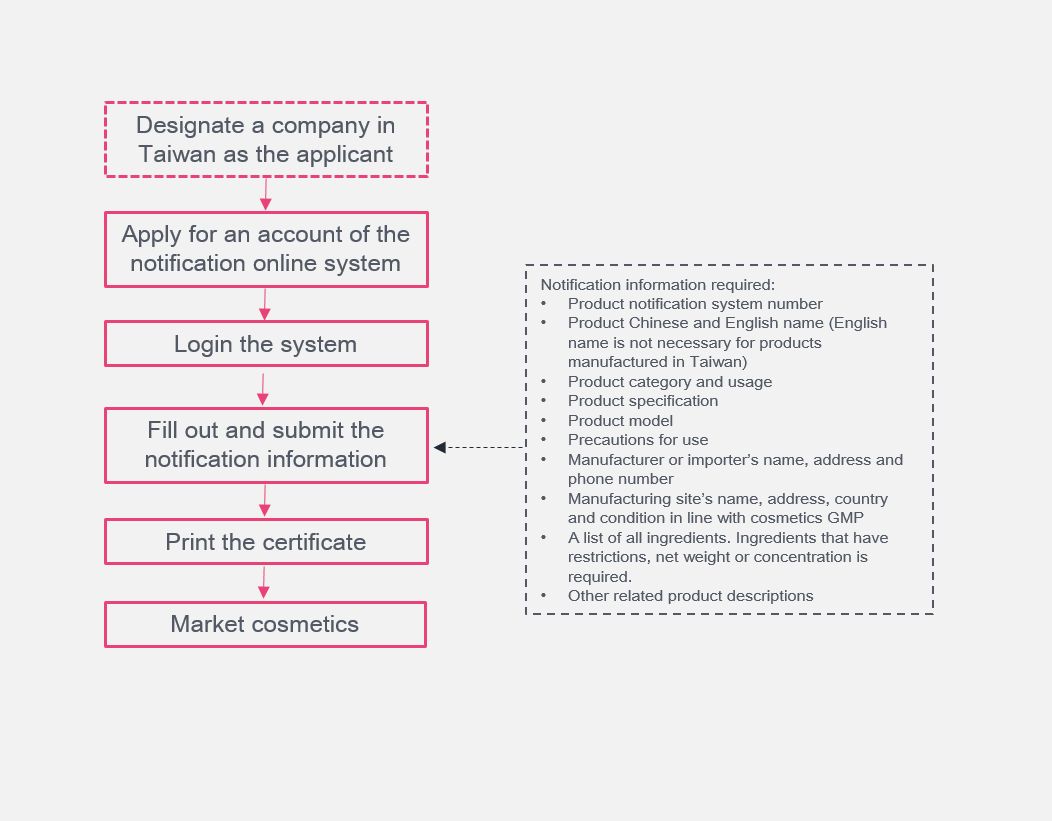 Notification Procedures
Notification Procedures
The TFDA established an online notification system https://cos.fda.gov.tw/TCAL/ for cosmetic companies to notify their products and print the notification certification. Applicants shall first contact TFDA to obtain an account and password for the system and then proceed with the notification. For now, notifications submitted online are free of charge.
If it is necessary to confirm whether the notification information is true, the competent authority will inspect the applicant's workplace or business place and check relevant supporting documents.
What is worth noting is that the notification shall be completed before the marketing of cosmetic products rather than the manufacture/import of cosmetics. Therefore, international companies can export directly without the need to submit a notification certificate for Custom clearance.
The validity period for product notification is 3 years. During the validity period, only products with ingredient changes require re-notification; for other circumstances, just a modification of notification is fine. Before the expiration date of notification, the applicant will receive a reminder email. The extension shall be completed within 3 months before the expiration date.
Product Information Files (PIF)
Cosmetic Hygiene and Safety Act stipulated that cosmetics manufacturers and importers shall complete the establishment of Product Information Files (PIF) before supply, sales, gifts, public displays, or trials for consumers.
The PIF dossiers include 16 documents, shown in the table below:
| No. | Required Document of PIF | Detailed Requirements |
| 1 | Product basic information (name, category, formulation, usage, name, and address of the manufacturer, importer’s information) | Include entrusted distribution certifications from overseas manufacturers |
| 2 | A notification certificate | Related data shall be consistent with the notification documents |
| 3 | Full ingredients and concentration | Require the name and concentration of every ingredient |
| 4 | Product label, instruction, outer packaging or container | Require clear labeling or instructions if there are other colors or types. |
| 5 | GMP certificate or self-commitment | |
| 6 | Manufacture method and process | Describe the method and process by texts or figures |
| 7 | Use method, application area, usage amount, frequency and target users | Include precautions for use |
| 8 | Adverse reaction data | Include adverse reaction records |
| 9 | Physical and chemical properties of products and ingredients | Include specifications of each ingredient and the product |
| 10 | Ingredients’ toxicological data | If there are new R&D or new usage ingredients, the safety data signatory shall assess appropriate safety testing |
| 11 | *Product stability test report | Including testing method, basis and reports |
| 12 | *Microbiological test report | Include microbiological specifications, testing method, testing reports |
| 13 | *Anticorrosion efficacy test report | Include testing method and testing report and supporting documents for low-risk products exempted from preservative efficacy testing |
| 14 | Functional assessment supporting data | Require supporting report or documents for products containing specific purpose ingredients or claiming special efficacy |
| 15 | Packaging materials information in contact with products | Include specification and volume of secondary packaging and related certifications or testing reports |
| 16 | Product safety data 1) safety assessment conclusions and advice signed by safety data signatory 2) certificate of safety data signatory’qualifications | 1) Include the assessment conclusion and advice, and signature date. 2) Name, education background and safety assessment training qualifications |
Note "*" : No.11-No.13 documents can be exempted if safety data signers clarify reasons according to product attribute or characteristics.
The PIF must be updated on a timely basis if any of the information above changes. For the language of PIF, both Chinese and English versions are acceptable, but if the original version is not in the two languages, a translation in either Chinese or English is required.
The manufacturer or importer shall keep the PIF (hardcopy or electronic) for future inspections for at least 5 years starting from when the product is first sold on the market. Generally, the competent authority will notify the enterprise 7 days before checking PIF.
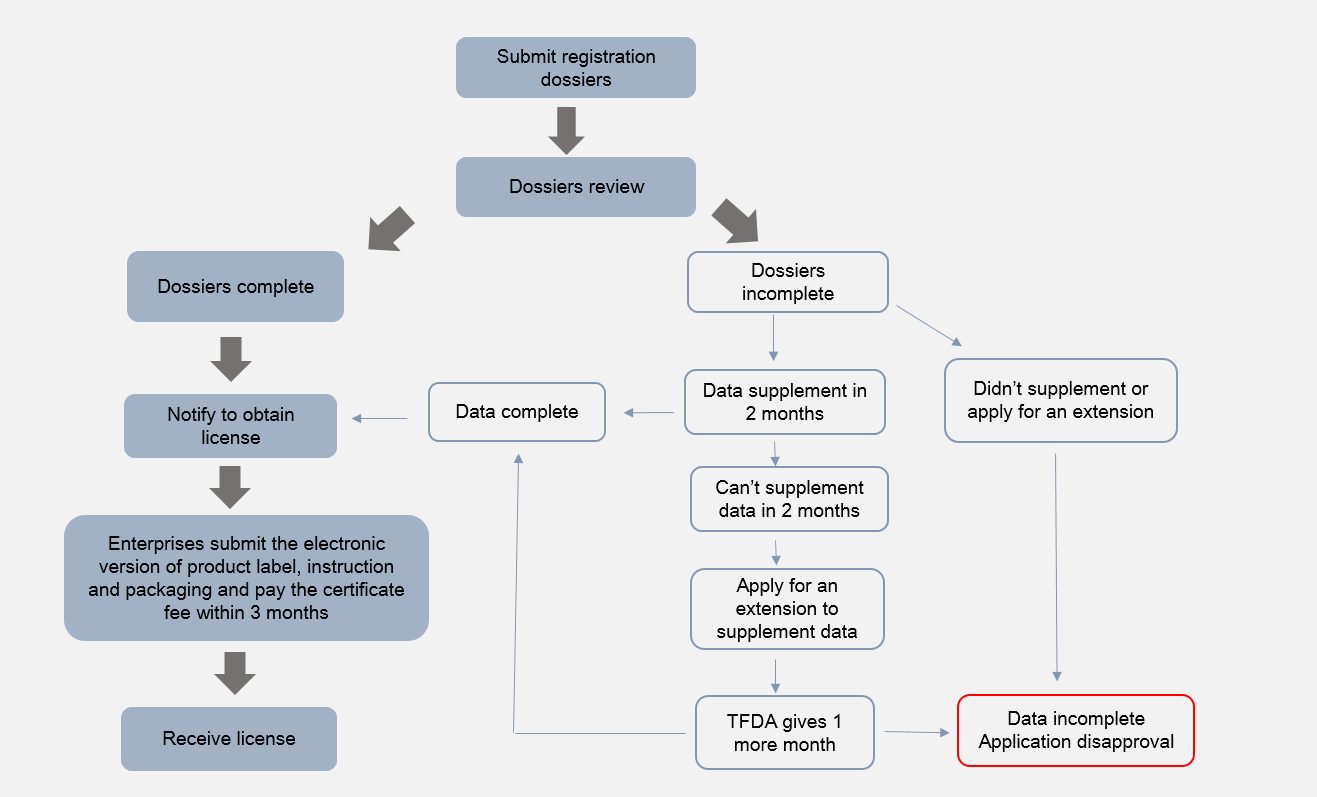
Specific Purpose Cosmetics Registration
Before July 1, 2024, specific purpose cosmetics are still subject to registration with the TFDA. The registration dossiers required and registration procedures are as follows:
Documents | Domestic | Imported |
Copy of business registration certificate | √ | √ |
Copy of pharmacist’s practicing license/resident cosmetic manufacturing supervisors’ certificate of qualifications and employment | √ | |
Samples of the product label, instruction and packaging design | √ | √ |
Testing report (including product characters, main ingredients, testing methods, quantitative methods, ingredients’ limitations, testing results, etc.) | √(can be replaced by GMP certificate) | √ |
For entrusted manufacturers, copies of applicant’s company/business registration certificate and entrustment contract | √ | |
Authorization certificate in the recent 2 years (issued by foreign manufacturers that agree on the applicant to import and sell products in Taiwan) | √ | |
Manufacture and sales certificate of country of origin in the recent 2 years | √ | |
The ingredient list in the recent 2 years | √ | |
For entrusted manufacturers, documents to explain the relationship between the entrusting party and the entrusted party. | √ | |
For those containing bovine and sheep tissue components, certificates proving that the product or ingredient do not come from the country/region or epidemic area of Bovine Spongiform Encephalopathy | √ |
After July 1, 2024, specific purpose cosmetics will be subject to simplified pre-market management that only a notification and establishment of PIF are required, just like general cosmetics.
4 Animal Testing
Starting from Nov 9, 2019, animal testing is banned in Taiwan unless one of the following conditions applies and has been approved by the central competent authority:
The ingredient is widely used, and its function cannot be replaced by other ingredients
Those that require animal testing to be conducted, having evaluation data that demonstrate the potential for harming human health
Cosmetics in violation of this provision shall not be sold.
5 Manufacturing Requirements
According to the new cosmetic regulatory framework, GMP will be mandatory for both manufacturers and importers. But the implementation date will be different for different cosmetic categories:
Cosmetic category | Implementation date |
Specific purpose cosmetics | July 1, 2024 |
Baby cosmetics, lip care cosmetics, eye care Cosmetics, non-medical toothpaste, and mouthwash | July 1, 2025 |
Other general cosmetics (solid handmade soap excluded) | July 1, 2026 |
Starting from the implementation date, corresponding cosmetic companies shall comply with the new GMP regulation-Cosmetic Good Manufacturing Practice Regulations. A domestic manufacturer complying with this GMP can submit the following documents to apply for a GMP compliance certificate:
Application form
Applicant's copy of company/business registration certificate
A copy of the factory registration certificate
After receiving the application, the competent authority will conduct an on-site inspection and issue the certificate to the qualified manufacturers.
GMP will also be an essential requirement for pre-market approval of both domestic and imported cosmetics. The information relating to the compliance status of GMP is required to submit for notification, and the GMP certificate or self-commitment is necessary for PIF. For registration of domestic specific purpose cosmetics, if the manufacturer has a GMP certificate, he can be exempted from submission of product testing reports. But the exemption rule is not applicable for imported specific purpose cosmetics.
As for overseas companies, it is also worth noting that TFDA also accepts ISO 22716 or equivalent regulations, and TFDA may conduct on-site inspections to overseas manufacturing sites to confirm the compliance status if necessary.
6 Labeling & Claims & Advertisements
The new regulations also update the information required on labels, shown in the table below:
Type | From July 1, 2021 |
Information required on labels | l Product name l Function l Usage and storage instructions l Net weight, volume, or amount l Full ingredient names. For specific purpose cosmetics, the content of specific purpose ingredients contained therein shall be labeled separately l Precautions for use l Name, address, and telephone number of manufacturer or importer, country of origin of imported product l Manufacturing date and shelf life, or manufacturing date and expiration date, or shelf life and expiration date; l Lot number l Other information required to be labeled |
Starting from July 1, 2021, cosmetics imported but re-packaged in Taiwan shall mark the words “臺灣分裝” or “臺灣改裝”( "re-packaged in Taiwan" or "sub-packaged inTaiwan") on the outer packaging or container. If imported cosmetics have "drug", "quasi drug", "medicated" or similar words on the outer packaging or container and are regulated in the places (countries) of origin or countries of sale as OTC drugs or quasi drugs, a notice should be labeled on the outer packaging or container, which is "this product is a cosmetic without medical effect".
Advertisement
Taiwan's existing pre-market approval of advertisements has been invalidated according to explanation No. 744 of Grand Justice. And according to Regulations Governing of Criteria for the Label, Promotion and Advertisement of Cosmetic Products Identify False, Exaggerated or Having Medical Efficacy, cosmetics advertisements, labels or claims shall not be exaggerated, fake or related to medical efficacy. If a product advertisement violates related regulations, the manufacturer will be subject to fines. Severe violations of the regulations are subject to product recall and rectification of all future advertisements.
7 Post-market Surveillance
Compared to the previous cosmetic regulation system in Taiwan, the new system focuses more on post-market surveillance and strengthens enterprises' obligations:
Establish and maintain source and flow data of products (products directly sold to consumers excluded)
Report the serious adverse effects
Notify sellers and recall incompliant products from the market
Criminal liability for illegal cosmetic operation activities is abolished while fines are increased
Cosmetics businesses are subject to random sampling checks and shall give their cooperation, and shall not evade, obstruct, or refuse inspection.
Part 3 Ingredients
1 General Requirements
On May 30, 2019, the TFDA released 6 brand-new ingredient lists to regulate the usage of cosmetic ingredients and conform to the new regulatory framework, among which 4 ingredient lists were just implemented on Jan 1, 2020.
| List Name | Release Date | Implementation Date |
| List of Ingredients Prohibited in Cosmetic Products | 2021-06-17 | 2021/7/1 |
| List of Preservatives in Cosmetic Products | 2019/12/5 | 2020/7/1 |
| List of Specific Purpose Ingredients in Cosmetic Products | 2019/5/30 | 2020/1/1 |
| List of Micro-organisms Limits in Cosmetic Products | 2019/5/30 | 2020/1/1 |
| List of Micro-organisms Limits in Cosmetic Products (To Be Enforced) | 2021/9/7 | 2022/1/1 |
| List of Ingredients Restricted in Cosmetic Products | 2019/5/30 | 2020/1/1 |
| List of Legal Colorants in Cosmetics | 2020/9/29 | 2021/7/1 |
2. Specific Purpose Ingredients
Specific purpose ingredients, the active ingredients contained in specific purpose cosmetics, shall be those in the List of Specific Purpose Ingredients in Cosmetic Products and comply with the corresponding requirements for purpose and maximum concentration.
If the specific purpose ingredient used is a new substance with a new purpose or new maximum concentration, the applicant shall attach relevant information stipulated in the table below:
Items | Scope of Documents | New Ingredient | New Usage | New Limit (Improving Use Concentration) |
Origin and Discovery Process, Application Situation at Abroad | Origin and Discovery Process | ○ | ○ | ○ |
Application Situation at Abroad | ○ | ○ | ○ | |
Properties Comparison | ○ | ○ | ○ | |
Physical and Chemical Properties, Testing Specification and Testing Methods | Chemical Constitutional Formula | ○ | X | X |
Physical Constitutional Formula | ○ | X | X | |
Testing Specification and Testing Methods | ○ | ○ | ○ | |
Stability Testing | Long-term Testing | ○ | ○ | ○ |
Stress Testing | ○ | ○ | ○ | |
Accelerated Testing | ○ | ○ | ○ | |
Safety Testing | Acute Toxicity Test | ○ | △ | △ |
Subacute Toxicity Test | ○ | △ | △ | |
Chronic Toxicity Test | ○ | △ | △ | |
Local Irritation Test | ○ | △ | △ | |
Antigenicity Test | ○ | △ | X | |
*Mutagenicity Test | △ | X | X | |
*Carcinogenicity Test | △ | X | X | |
*Reproductive Toxicity Test | △ | X | X | |
Absorption, Distribution, Metabolism and Excretion Tests | Absorption Test | △ | △ | △ |
Distribution Test | △ | △ | △ | |
Metabolism Test | △ | △ | △ | |
Excretion Test | △ | △ | △ | |
Related Usage Documentation | Function or Efficacy Certificate | ○ | ○ | ○ |
Human Trial Reports | ○ | ○ | ○ | |
Approval Documentation from other countries | △ | △ | △ |
Note 1:"○" means mandatory attached documentation; "△" means the requirement to attach depends on each individual case; "X" means there is no need to attach the documentation.
Note 2: For "Application Situation at Abroad," if products are from their own research and development, the documents are not required.
Note 3: For "Testing Specification and Testing Methods," ingredients and preparations are included.
Note 4: For "Stability Testing and Safety Testing," ingredients and preparations are included. The items with "*" are exempted from attaching reports of preparations.
Note5: Antigenicity Tests include skin allergy tests, photosensitization tests, etc. Local irritation tests include skin irritation tests and mucosa irritation test.
Note 6: Stability testing must be conducted in line with international standards or Standards for Stability Testing of Drugs released by TFDA.
Note 7: Cosmetic stakeholders intending to conduct safety assessments of cosmetics or cosmetic ingredients within Taiwan shall refer to provisions specified in Paragraph 4 and 6 of Article 6 of Cosmetic Hygiene and Safety Act.
However, there is an exemption. If the incompliant specific purpose ingredient has been permitted for use in any of the countries/regions recognized by the TFDA such as EU, USA or Japan, the ingredient can also be permitted for use in Taiwan but not exceed the limitation set by the country/region. In such a case, the applicant shall submit the local approval documents/certificates instead when applying for the registration.



