Japanese cosmetics are regulated under Pharmaceutical and Medical Devices Law (PMDL, formerly Pharmaceutical Affairs Law) supported by a series of subsidiary rules, standards and guidance documents issued by the competent authority, Ministry of Health, Labour and Welfare (MHLW).
Japan legally classifies cosmetics (in the broad sense of beauty products) into two categories: cosmetics and quasi drugs. The regulations governing each category differ greatly.
Part 1 Regulatory Framework and Competent Authority
1 Existing Key Cosmetic Regulations in Japan
2 Competent Authorities
Ministry of Health, Labour and Welfare is the competent authority regulating cosmetics and quasi drugs, which is responsible for formulating cosmetic and quasi drug regulations and standards.
Pharmaceutical Affairs and Food Sanitation Council is MHLW’s back-up expert panel.
Pharmaceuticals and Medical Devices Agency (PMDA) focuses on review applications of quasi drugs and cosmetics (foreign manufacturer, importer) notifications, and evaluate the adverse effect reports.
Prefectural governments are responsible for licensing, inspection and guidance.
Part 2 Cosmetics
1 Definition
Japan defines cosmetics as “articles with mild action on the human body, which are intended to be applied to the human body through rubbing, sprinkling or other methods, aiming to clean, beautify and increase the attractiveness, alter the appearance or to keep the skin or hair in good condition.” There are 6 categories in total:
Categories | Products |
Perfume and eau de cologne | Perfume and eau de cologne |
Makeup cosmetics | Foundation creams, lipsticks, eye makeup and others |
Skin care cosmetics | Skin lotion, essence, skin milk, cleansing cream and others |
Hair care products | Shampoo, hair treatment and others |
Special purpose cosmetics | Sunscreen, shaving cream and others |
Cosmetic soaps | Soaps for cosmetics |
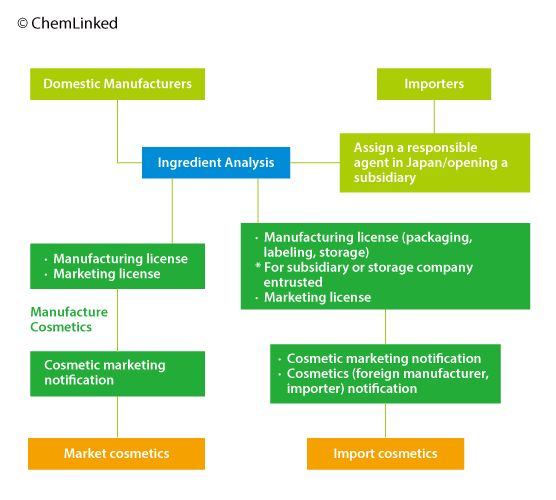
2 Approval Procedure of Cosmetics
2.1 Ingredients Analysis
Marketing license holders take full charge for ingredient safety problems. When considering selling products in Japan, they are recommended by the authority to conduct ingredients analysis to ensure conformity with Japanese cosmetic ingredient regulations although the analysis is not mandatory and there is no need to submit related reports prior to marketing. But related reports will be inspected if their products are found unsafe.
This analysis is performed on samples by “testing and inspection facilities” designated by the MHLW, owned or contracted by manufacturers/importers. The following items are to be tested:
Preservatives,
UV absorbents,
Anti-oxidants,
Heavy metals,
Japanese legal color index colorants,
Prohibited ingredients.
Also, some specific tests could be performed, such as pH, viscosity, specific gravity, bacterial count, patch tests, stability tests and more. As there are numerous testing items, the MHLW provides a checklist for manufacturers/importers for confirmation.
2.2 Comparison of Two Licenses
Items | Cosmetic Manufacturing License | Cosmetic Marketing License |
Classification | 1. Manufacturing license (general) 2. Manufacturing license (packaging, labeling, storage) | No |
Qualification | 1. Manufacturing license (general)
2. Manufacturing license (packaging, labeling, storage)
*Not permitted to market cosmetics |
*Not permitted to conduct activities of manufacturing, packaging, labeling and storage. |
Requirements |
|
|
Application dossiers |
|
|
Authority | Prefectural governments | Prefectural governments |
Validity | 5 years | 5 years |
2.3 Good Quality Practice (GQP) and Good Vigilance Practice (GVP)
As part of the GQP, marketing license holders are required to properly evaluate their production management and quality control of cosmetics to be marketed. A procedures manual is used as reference for the registration of product delivery, collection of information on the quality of products, processing of defective products, products’ recall from the market, etc. This standard aims at maintaining the quality of products that are marketed by the license holder.
Additionally, marketing license holders must establish systems that are capable of providing and retaining accurate information in response to consumer inquiries along with a monitoring system that handles customer complaints on product quality and product recalls, as required by the GVP standards. This requires collection of information relating to the safety of products provided by the competent authorities, professional organizations, manufacturers, retailers, consumers, researchers, etc. After analysis of this information and if deemed necessary (possibility of harmful effects caused by the products, for example), the marketing license holders may undertake corrective actions such as the recall of products from the market or amending package warning and precaution labels. Furthermore, if the license holder becomes aware of any information indicating that one of the cosmetic products may have a harmful effect, they must report that fact to the MHLW within 30 days.
2.4 Notifications
After obtaining the licenses but before initiating marketing or importation, manufacturers are required to submit cosmetic marketing notification while importers shall submit cosmetics (foreign manufacturer, importer) notification in addition to the marketing notification.
Notification | Administrative agency Responsible |
Cosmetic marketing notification | Same prefecture as that which has granted the cosmetics marketing license |
Cosmetics (foreign manufacturer, importer) notification | Pharmaceuticals and Medical Devices Agency, Japan (PMDA, Tokyo) |
In the past the documents listed above required simultaneous submission however since Jan 1 2016 the requirement is abolished. The two notifications must either be accompanied by a list of full ingredients from the importer’s supplier or manufacturer or, if this list cannot be obtained, a record of the testing and inspection results confirming the product does not contain any prohibited ingredient combinations instead.
3 Labeling & Claims
Cosmetic labels are required to be in Japanese and must be clearly and explicitly listed. Labeling with false or potentially misleading expressions, and unapproved claims of functionality or efficacy are prohibited.
3.1 Required Labeling Information
Company name and address
Registered Japanese product name
Batch No. or Batch Code
Shelf life
Full ingredient labeling in Japanese
Country of origin
Warning; Stop using, if irritation occurs
Other information required to be labeled
3.2 Claims Allowed and Prohibited
With respect to claims, Japan implemented a list of allowed efficacy claims for cosmetics, which include 56 wordings. Please click here to see the list.
The following claims are prohibited:
Anti-ageing, rejuvenating
Lifting
Claims like pharmaceuticals
Stress easing
Doctor’s name, recommendation and development
Genetic effects
Safety: assuring expression on safety is prohibited, even if they are safety
Efficacy: assuring expression on efficacy is prohibited; numerical expression, %, numbers, photos before and after use
3.3 Full Ingredient Labeling
Full ingredient labeling in Japanese is mandatory on outer package of cosmetics but can be omitted from primary package. Both outer and primary package must be the same information except full ingredient labeling.
The detailed requirements for full ingredient labeling are:
Language: Japanese
All non-color ingredients in descending order of predominance.
Followed by all colors and non-color ingredients of which content is 1% or less in random order.
No need to indicate incidental ingredients.
A mixed ingredient must indicate each individual ingredient.
An extract is indicated to be divided an extract and a solvent or a diluted solution.
Perfume can be indicated “perfume”.
The Japan Cosmetic Industry Association (JCIA) has compiled a Japanese version of the "List of Cosmetic Ingredient Label Names" to be used in conjunction with the PMDL's requirements to list all ingredient names on the labeling. Companies can refer to the document to standardize the Japanese name of ingredients.
Part 3 Quasi Drugs
1 Definition
Quasi drugs are defined as articles for the purpose of preventing nausea and other discomfort, preventing heat rash, soreness, etc., encouraging hair growth or removing hair or exterminating and preventing mice, flies, mosquitoes, fleas, etc. Generally the quasi-drugs include:
| No | Scope | Remark |
| 1 | Deodorants | |
| 2 | Hair growth treatment | |
| 3 | Depilatories | |
| 4 | Hair dyes | |
| 5 | Permanent waving agent | |
| 6 | Bath products | |
| 7 | Dentifrice | |
| 8 | Medicated cosmetics |
|
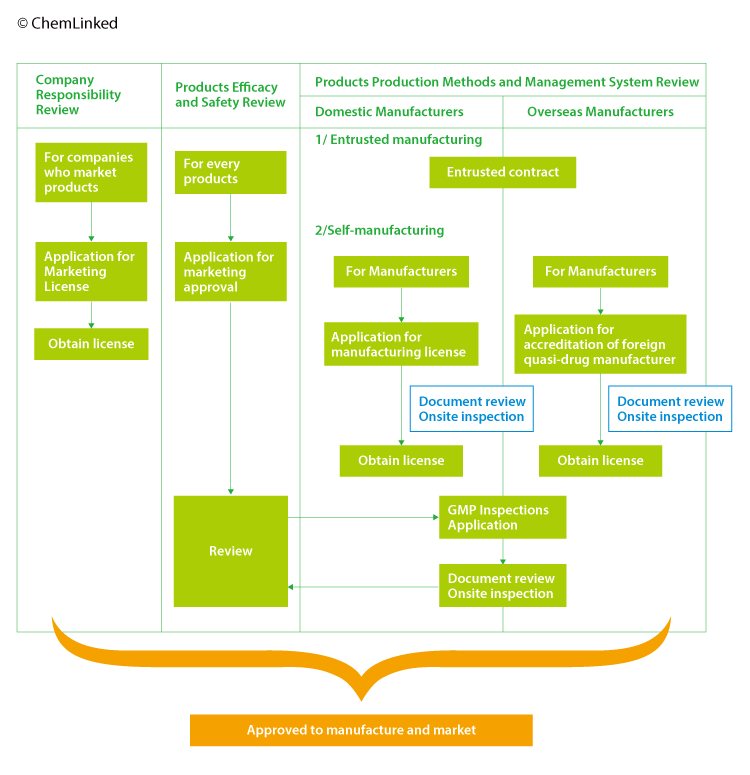
2 Approval Procedures
Note 1: The application procedures of marketing license and manufacturing license for quasi drugs are similar with cosmetics. But the requirements for quasi drugs are more strict.
Note 2: Manufacturing license of quasi drugs includes three categories:
Sterile manufacturing
Packaging, labeling and storage
General (except the above categories)
Repacking (changing quasi drugs in bulk to cosmetics in small container or in bags) do not fall into the second category. One manufacturer can obtain all the three kinds of licenses.
Note 3: As imported quasi drugs require storing for testing at the Customs, importers’ subsidiary or storage company entrusted shall obtain a manufacturing license (packaging, labeling and storage).
2.1 Marketing Approval
The marketing approval is to ensure the quality, efficacy and safety of each quasi drug. Domestic manufacturers can submit the application to either MHLW or prefectural governments while importer can only submit to MHLW. The application form for importers requires company names and addresses of both importer and domestic marketing license holder.
Information requiring submitting | Disapproved situations |
| 1) The applicants didn’t obtain the marketing license. 2) The manufacturer didn’t obtain the manufacturing license 3) In the phase of review quality, efficacy and safety, either of the following circumstance occurs:
4) The manufacturing does not comply with GMP and GQP. |
2.2 Accreditation of Foreign Quasi-drug Manufacturer
A foreign manufacturer intending to manufacture quasi-drugs in foreign countries and export them to Japan is required to be accredited by the MHLW as an “Accredited Foreign Manufacturer”. A Japanese marketing license holder who markets quasi drugs manufactured by a foreign manufacturer can make an accreditation application on the manufacturer’s behalf. The MHLW has an authority to grant accreditation to a foreign manufacturer, while PMDA examines buildings and facilities of the manufacturing establishment for accreditation. But the application shall be submitted to PMDA for processing.
Categories of Accreditation
The accreditation is granted for each manufacturing establishment according to the category specified by the Enforcement Regulations. There are three categories:
Accreditation for all or part of the manufacturing process of sterile quasi drugs (excluding manufacturing processes indicated in Item 3)
Accreditation for all or part of the manufacturing process of quasi drugs other than those indicated in the preceding item (excluding manufacturing processes indicated in the next item)
Accreditation for only the process of packaging, labeling or storage among the manufacturing processes of quasi drug
Required Documents for Application:
Explanation if the applicants don’t comply with the No.3 Article 5 Pharmaceutical law.
The curriculum vitae for the responsible person at the manufacturing establishment
List of product(s) to be manufactured (a list of product(s) to be exported to Japan is acceptable) and documents on the manufacturing process
Documents on the buildings and facilities of the manufacturing establishment
Documents on the category of radioactive drugs and related manufacturing equipment
Copies of licenses or certificates relating to manufacturing and marketing manufacturers obtained in their country
2.3 GMP Inspection
For GMP inspection, PMDA conducts on-site and document- based inspections of domestic and foreign manufacturing sites for products classified as “high-risk” (beauty products include hair dyes, hair perm agents and bath agents), in order to ascertain whether their manufacturing facilities and manufacturing and quality controls comply with standards such as the Good Manufacturing Practice (GMP), and whether the manufacturing sites have a system for manufacturing products of adequate quality. It is a mandatory requirement for manufactures who intending to obtain marketing approval, manufacturing license and accreditation of foreign quasi-drug manufacturer.
Standard administrative processing time required for GMP compliance inspection at PMDA is 6 months. Applications are to be submitted at an appropriate timing with consideration of the progress of the review for marketing approval.
Documents required to be submitted
1) Documents specified in Enforcement Notifications
A copy of notification of GMP compliance inspection results or GMP compliance inspection result report of GMP inspection (including inspections conducted by other authorities) conducted over the past 2 years from the application date of the compliance inspection
For the inspections of a foreign manufacturing sites, a GMP compliance certificate issued by a country; a certificate issued by a country; or a WHO certificate or a GMP compliance certificate issued by the authority of a country
A copy of the application form for marketing approval of the product whose application is being submitted (or a copy of a manufacturing notification of drugs for export)
2) “Documents required by the compliance inspection authority” specified in Chapter 1, Section 3-9, (2), (d) in Enforcement Notifications
Outline of the product, etc. subject to inspection and outline of the manufacturing site
Outline of the product subject to the inspection at the manufacturing site (Form 1)
Outline of the drug manufacturing site “for manufacturing sites in Japan” (Form 3) or outline of drug manufacturing site “for manufacturing sites in foreign countries” (Form 3). Applications pertaining to external testing institutions do not require submission of Form 2 or Form 3; submit Form 1 filled out the necessary columns for the external testing institutions.
Checklist 1 should be filled out and attached to the inspection application form along with the above documents.
3 Labeling & Claims
The required labeling information for quasi drugs is as follows:
Marketing license holder name and address
Product name
Batch No. or Batch Code
Weight, volume or content
Active ingredient name and concentration
For quasi drugs containing specific ingredients prescribed by the MHLW, indicate the ingredient ingredient name
Shelf life
Warning; Stop using, if irritation occurs
Word of quasi-drug
Mark the word of quasi-drug in accordance with the requirements of MHLW
Other information required to be labeled by MHLW
MHLW doesn't offer a reference list of permitted or prohibited claims for quasi drugs. The claims of quasi-drugs are based on active ingredients contained and can only be marked after getting an approval.
e.g.) Whitening efficacy: The product must contain a whitening active ingredient
Anti-acne efficacy: The product must contain an anti-acne active ingredient
Part 4 Advertisement
Standards for Advertisements of Drugs, Quasi Drugs, Cosmetics and Medical Device” detailed the prohibited claims in advertisements.
Type | Prohibited claims |
Product Name | Names other than the permitted names specified in Article 12, 18 and 22 of PMDL |
Claim of efficacy |
|
| Claims causing over-consumption and misuse |
| Libeling other companies |
| Claims like “recommended by medicine, cosmetologist, etc. or appointed, recognized or recommended by a specific well-known institution” |
| Expressions creating an uncomfortable feeling |
| Advertisements in TV, radio programs, movies and TV series explicitly or implicitly expressing products’ efficacy and function or misleading consumers |
Part 5 Ingredients
Cosmetics and quasi drugs ingredients are mainly subject to the following standards separately:
Standards for Cosmetics
Japanese Standards of Quasi-drug Ingredients 2006
The ingredients of both categories also require complying with Standards for Ingredients of Biological Origin and Ordinance on Tar Color Used in Pharmaceuticals.
1 Cosmetic Standard
The Standards for Cosmetics (Ministerial Notification in September 2000) include the lists of prohibited or restricted ingredients for use in cosmetics (positive list or negative list). Preservatives, UV absorbers and tar colors are subject to a positive list that indicates the maximum limits. All other ingredients may be used in cosmetics after safety verification and selection, except those included in the negative list.
List | Number of Ingredients |
30 | |
48 | |
33 | |
19 |
2 Japanese Standards of Quasi-drug Ingredients 2006 (JSQI)
JSQI is the overarching technical standards for quasi drugs, which includes the ingredients allowed for use in quasi-drugs, defines their specifications and introduces testing methods. Apart from the JSQI, MHLW also introduced the following documents to regulate active ingredients and additives used in quasi-drugs.
Types | Lists |
Additives | Permitted Additives in Quasi Drugs
|
Active Ingredients | |
Permitted Active Ingredients in Medicated Tooth-cleansing Products |
Note 1: Additives refer to substances other than active ingredients in preparations, such as excipients, stabilizers, emulsifiers, buffer agents, adhesives, colorants, fragrances, corrective agents, etc.
Note 2: Ingredients in the above list are regarded as existing ingredients.
For ingredients exceeding the limit set in the above lists or which meet the following criteria, documents regarding its efficacy, safety, and ingredient formulation, purpose, etc. require submission for review according to the requirements below.
 Note: o means mandatory attached documentation; x means to attach or not to attach depends on each individual case; △ means there is no need to attach the documentation in principle but documents are likely to be submitted based on the application of products.
Note: o means mandatory attached documentation; x means to attach or not to attach depends on each individual case; △ means there is no need to attach the documentation in principle but documents are likely to be submitted based on the application of products.
Items | Specific documents |
I Origin and Discovery Process, Application Situation at Abroad | 1. Origin and Discovery Process |
2. Application Situation at Abroad | |
3. Properties Comparison, or Comparison with Other Quasi Drugs | |
II Physical and Chemical Properties, Testing Specification and Testing Methods | 1. Chemical Constitutional Formula |
2. Physical Constitutional Formula | |
3. Testing Specification and Testing Methods | |
III Stability Testing | 1. Long-term Storage Testing |
2. Stress Testing | |
3. Accelerated Testing | |
IV Safety Testing Reports | 1. Single-dose Toxicity Study |
2. Repeated-dose Toxicity Study | |
3. Genetic Toxicity Study | |
4. Carcinogenicity Study | |
5. Reproductive Toxicity Test | |
6. Local Irritation Test | |
7. Skin Sensitivity Test | |
8. Photo Safety Test | |
9. Absorption, Distribution, Metabolism and Excretion Tests | |
10. Human Patch Test | |
11. Long-term Dose (Safety) Test | |
V Efficacy and Function Reports | 1. Supporting Documentation of Efficacy and Function |
2. Human Trial Reports |
The registration duration for additives is around one to two years while for active ingredients at least 5 years are required.